Graphical Representations of the Gas Laws
- The relationship between any two of the variables which describe the properties of an ideal gas can be established by keeping the other variables constant
- These variables are:
- Pressure
- Volume
- Temperature
- Amount of gas (moles)
- These relationships expressed mathematically and graphically are known as Gas Laws
- These variables are:
Pressure-Volume Relationship: Boyle’s Law
- This relationship was first investigated by the British chemist, Robert Boyle, using a J-tube, showing that the volume of gas decreased as the pressure increased
- This observation is summarized by the statement by Boyle’s Law which states that: the volume of a fixed amount of gas at constant temperature is inversely proportional to the pressure:
V ∝ P ; PV = constant
- Simplifying the mathematical equation gives Boyle’s equation:
P1V1 = P2V2
Pressure-Volume Relationship

Boyle’s experiment uses a J-tube to demonstrate the volume-pressure relationship of a gas. Gas volume decreases on increasing the pressure in the tube
- This relationship can also be visualized using two graphs:
- A plot of V against P gives a curve for a given quantity of gas at a fixed temperature
- A straight line is obtained when V is plotted against 1/P or P against 1/V
Graphical Representation of Pressure-Volume Relationship

Graphical representations of Boyle’s Law
Temperature-Volume Relationship: Charles’s Law
- The earliest investigator of the effect of temperature on volume was French scientist, Jacques Charles
- He observed that at constant pressure, the volume of a gas sample expands when heated and contracts when cooled
- At any given pressure, the plot of volume versus temperature yields a straight line
- By extrapolating the line to zero volume, we find the intercept on the temperature axis to be -273.15 ℃
Temperature-Volume Relationship on Celsius Scale
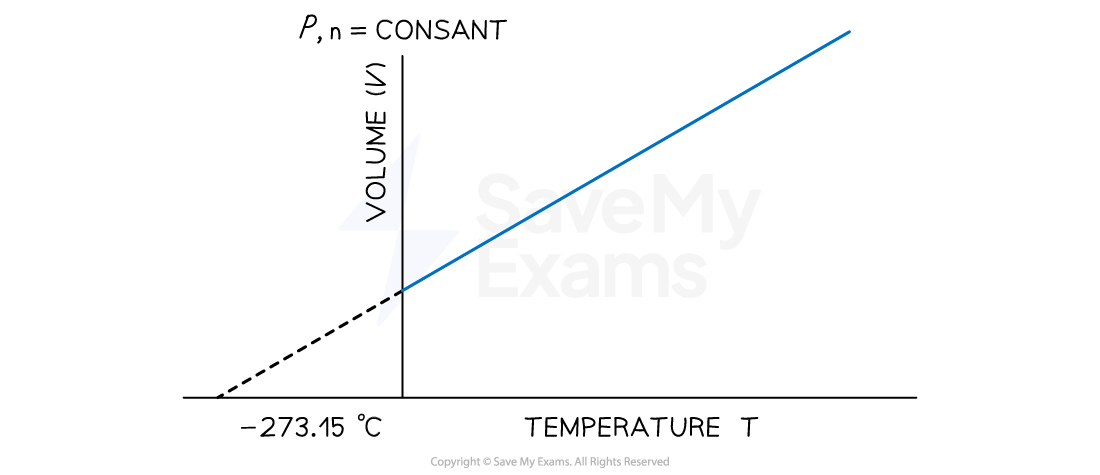
A graph showing the linear relationship between temperature (in degrees Celsius) and volume. The line intercepts the temperature axis at a value of 273.15 ℃
- This implies that at a temperature of 273.15 ℃, the volume of a substance, if it remains gaseous will be zero
- However, this could not happen, given that gases liquefy before this temperature, and the relationship does not apply to liquids
Kelvin Temperature Scale
- In 1848, British physicist William Thomson, whose title was Lord Kelvin, realized the significance of the volume-temperature relationship using the Celsius temperature scale
- He identified the lowest possible temperature as absolute zero
- He then set up an absolute temperature scale, now called the Kelvin temperature scale (K) with absolute zero as the starting point
- On the absolute temperature scale, 0 K is equivalent to -273.15 ℃
- In terms of the Kelvin scale, the temperature-volume relationship known as Charles’s Law can be stated as: the volume of a fixed amount of gas maintained at constant pressure is directly proportional to its absolute temperature:
V ∝ T or V/T = constant
- Simplifying the relationship gives:
V1/T1 = V2/T2
- A plot of volume against temperature in Kelvin also gives a straight line which intercepts the volume and temperature axis at zero
Temperature-Volume Relationship on the Kelvin Scale

A graph showing the linear relationship between absolute temperature and volume. The line intercepts the temperature axis at a value of 0 K
Quantity-Volume Relationship: Avogadro’s Law
- The relationship between the amount in moles of a gas and its volume follows from the work of Joseph Louis Gay-Lussac and Amedeo Avogadro
- Gay-Lussac observed that at a given pressure and temperature, the volumes of gases that react with one another are in the ratios of small whole numbers
- For example, two volumes of hydrogen gas react with one volume of oxygen gas to form two volumes of water vapor:
2H2 (g) + O2 (g) → 2H2O (g)
- A few years later, Amedeo Avogadro interpreted Gay-Lussac’s observation by proposing an important hypothesis which set the foundation for demonstrating the mole-volume relationship and Avogadro’s Law
- The hypothesis states that: Equal volumes of gases at the same temperature and pressure contain equal numbers of molecules
- For example, at 0 ℃ and 1 atm, 22.4 L of a gas contains 6.022 × 1023 gas molecule which is equivalent to one mole
- Avogadro’s law follows from this hypothesis: the volume of a gas maintained at constant temperature and pressure is directly proportional to the number of moles of the gas:
V ∝ n ; V/n = constant
- Simplifying the relationship gives:
V1/n1 = V2/n2
Quantity-Volume Relationship
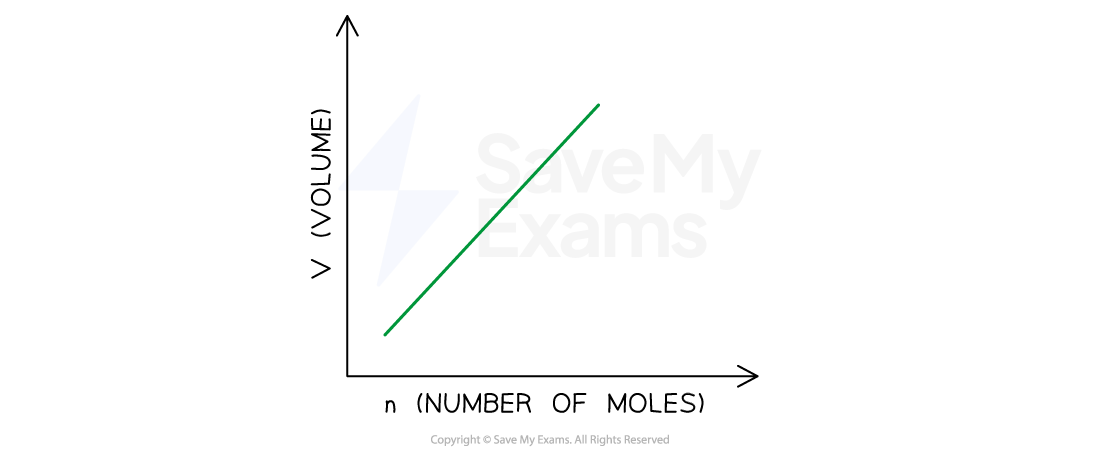
Graphical representation of Avogadro’s Law
Worked example
A chemical reaction produced 4.38 mL of hydrogen gas at 19 ℃ and 200 kPa pressure.
What volume of the gas would be produced at 25℃ at the same pressure?
Answer:
Analyze: From the question, the following parameters were provided
V1 = 4.38 mL
T1 = 19 ℃
T2 = 25 ℃
P = 200 kPa
We are then asked to calculate the final volume while pressure is kept constant
Plan: Since the pressure remains constant, the problem requires the use of the volume-temperature relationship expressed by Charles’s equation
Solution:
Step 1: Convert temperature values in ℃ to K:
T1 = 19 + 273 = 292 K
T2 = 25 + 273 = 298 K
Step 2: Rearrange Charles’s equation in terms of the final volume V2 and solve for V2:
V2 = V1 × T2 / T1
V2 = 4.38 × 298 / 292
V2 = 4.47 mL
Worked example
In a reaction involving the decomposition of limestone, CaCO3, 20.0L of CO2 at 23 ℃ and 1.00 atm were collected.
What would be the volume of CO2 collected if the pressure were halved at the same temperature?
Answer:
Analyze: We have the following parameters from the question:
P1 = 1.00 atm
V1 = 20.0 L
P2 = ½ P1 = 0.500 atm
T = 23 ℃
We are then asked to calculate the final volume while the temperature is kept constant
Plan: Since temperature is kept constant, the volume-pressure relationship using Boyle’s equation would be required to determine the new volume
Solution: Rearrange the Boyle’s equation in terms of V2 and solve for V2:
V2 = P1 × V1 / P2
V2 = 1.00 × 20.0/ 0.5
V2 = 40.0 L
Worked example
Sodium reacts with water at 25.0 ℃ to produce hydrogen gas. The equation of the reaction is given as follows:
2Na (s) + 2H2O (l) → 2NaOH (aq) + H2 (g)
246 mL of hydrogen gas is collected over water at 25.0 ℃ and 1.00 atm.
Calculate the mass of sodium used in the reaction. Vapor pressure of water at 25.0 °C is 0.0313 atm
Answer:
Analyze: This problem requires the application of stoichiometry, Dalton’s Law of partial pressure and the ideal gas equation.
Plan:
- Determine the pressure of the dry hydrogen gas produced, using Dalton's partial pressure equation
- Use the ideal gas equation to determine the number of moles of hydrogen obtained
- Use the stoichiometric ratio from the balanced chemical equation to determine the number of moles of sodium which must have reacted
- Convert the number of moles of sodium to the mass of sodium
Solution
Step 1: Determine the pressure of the dry hydrogen gas produced:
- PH2 = Ptotat - PH2O
- PH2 = 1.00 - 0.0313
- PH2 = 0.9687 atm
Step 2: Determine the number of moles of hydrogen gas using the ideal gas equation:
- PV = nH2RT
- PH2 = 0.9687 atm
- VH2 = 246 mL/ 1000 = 0.246 L
- Remember: Volume must be in L
- R = 0.08206 L.atm mol-1 K-1
- T = 25 + 273 = 298 K
- Remember: Temperature must be in K
- nH2 = ?
- nH2 = PV/RT
- nH2 = 0.9687 × 0.246 / 0.08206 × 298
- nH2 = 0.00974 mole
Step 3: Determine the number of moles of sodium required given a 2:1 ratio from the balanced chemical equation
- nNa = 2 × nH2
- nNa = 2 × 0.00974
- nNa = 0.0195 mole
Step 4: Convert number of moles determined in step 3 to mass of sodium by multiplying moles by the molar mass of sodium:
- MNa = nNa × MrNa
- MNa = 0.0195 × 22.99
- MNa = 0.45 g

